the law of conservation of matter states that
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. The law of conservation of matter and energy states that matter is neither created nor destroyed but conserved.

Aw Of Conservation Of Mass Law Of Conservation Of Matter Physical Science Matter Middle School Science As Conservation Of Mass Exit Tickets Matter Science
Advertisement Advertisement Chelboy Chelboy Answerstays the same.

. Matter can change form through physical and chemical changes but through any of these changes matter is conserved. The law of conservation of matter says that in chemical reactions the total mass of the products must equal the total mass of the reactants. They can only rearrange the matter and energy. So the mass of the product equals the mass of the reactant.
This applies to chemical equations. So the mass of the product equals the mass of the reactant. State the law of conservation of matter. For example when wood burns the mass of the soot ashes and gases equals the original mass of the charcoal and the oxygen when it first reacted.
The Law of Conservation of Matter states that matter cannot be created or destroyed. Jelly Bean Equations The Law of Conservation of Matter states that matter cannot be created nor destroyed. Even though the matter may change from one form to another the same number of atoms exists before and after the changes take place. This number is called the coefficient.
Humans do not have the ability to create or destroy matter atoms or energy. What is a substances critical point. Reaction Grid 15 toothpicks 4 different colors of jelly beans 16128 4 of the respective colors DirectionsBalance the. What physical change of state is the opposite of boiling.
The law of conservation of mass describes and generalizes the observation that a sample of matter will not change in mass even if it undergoes a chemical reaction. The law of conservation of matter states that in any given system that is closed to the transfer of matter the amount of matter in the system stays constant. The law of conservation of matter states that in chemical reactions the total mass of the products must equal the total mass of the reactants in other words it must stay the same. This appears on the surface to be wrong when someone looks at the simple issue of what happens to matter when burned on Earth.
The Law of Conservation of Mass Matter is anything that has mass and takes up space. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. Coefficients A formula may begin with a number. However the law does not explain why this phenomenon occurs.
In a physical change substances can change form but the total mass remains the same. In a physical change substances can change form but the total mass remains the same. For example when wood burns the mass of the soot ashes and gases equals the original mass of the charcoal and the oxygen when it first reacted. It includes molecules atoms fundamental particles and any substance that these particles make up.
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. The law of conservation of mass states that matter is neither created nor destroyed in a chemical reaction in a nuclear reaction it. What are the two general categories for properties of matter. This means that the amount of atoms that undergoes the change reactants must be the same as the amount that is produced product.
The law of conservation of matter states that atoms involved in a physical or chemical change can neither be created nor destroyed. If there is no number then 1 is understood to be in front of the formula. The law of conservation of matter also known as the conservation of mass states that the amount of matter in a closed system never changes. What physical change of state is the opposite of sublimation.
The number of atoms of each type of element must be equal on both sides of an equation. Law of Conservation of Matter States that during a chemical reaction matter cannot be created or destroyed. For example when wood burns the mass of the soot ashes and gases equals the original mass of the charcoal and the oxygen when it first reacted.
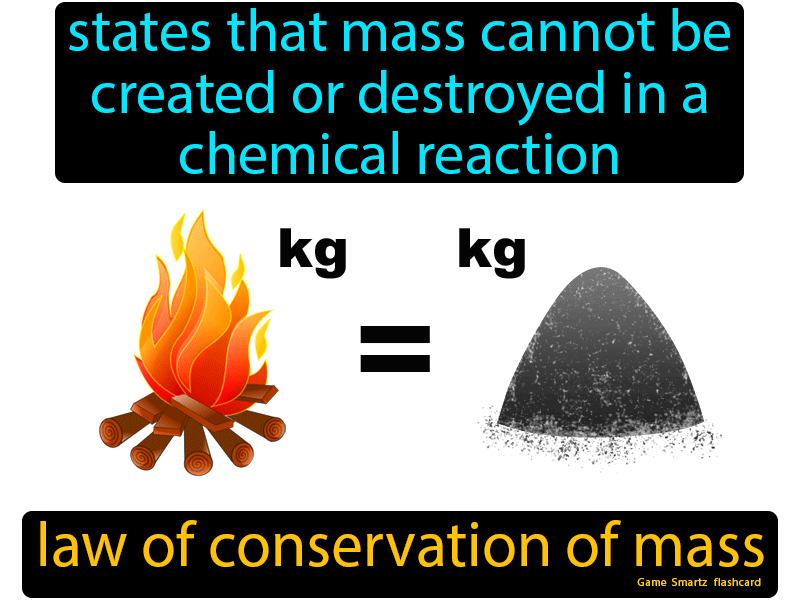
Law Of Conservation Of Mass Easy Science Conservation Of Mass Mass Activities Teaching Matter

Law Of Conservation Of Matter States That Mass Is Neither Created Nor Destroyed In Ordinary Chemical A Chemical And Physical Changes Physical Change Education
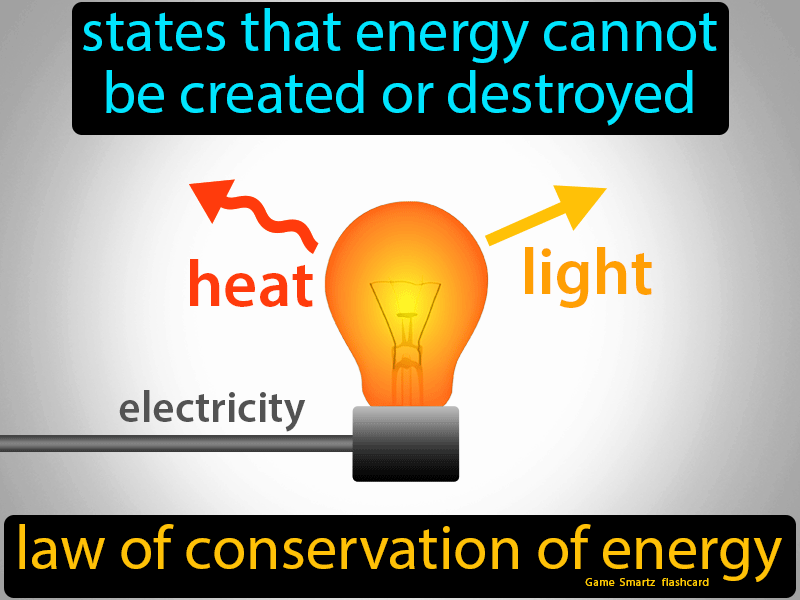
Law Of Conservation Of Energy Easy Science Chemical Energy Teaching Matter Energy

Law Of Conservation Of Matter Notes Interactive Science Notebook Interactive Science Science Notebook

Pin By Shannan Hildebrand On Teaching Ideas Conservation Of Mass Mass Activities Next Generation Science Standards
Post a Comment for "the law of conservation of matter states that"